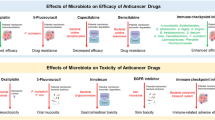
Abstract
Chemotherapeutic drugs play an important role in the treatment of cancer, but the individual differences of patients' sensitivity to chemotherapeutic drugs and the drug resistance of chemotherapeutic drugs have always been a thorny problem in clinical treatment. In recent years, with the progress in research on human microbiota, gut microbiome plays an increasingly important role in the diagnosis and treatment of diseases. Studies have shown that gut microbiota can regulate the tumour microenvironment and affect the efficacy and toxicity of chemotherapy through a variety of mechanisms. This paper focuses on the specific mechanism that gut microbiota uses to influence chemotherapy and the potential therapeutic effect of supplementing with probiotics, to provide an important basis for individualised treatment strategies to be used when treating malignant tumours.
Similar content being viewed by others
References
Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14. https://doi.org/10.1038/nature11234.
Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14(8):e1002533. https://doi.org/10.1371/journal.pbio.1002533.
Chow J, Tang H, Mazmanian SK. Pathobionts of the gastrointestinal microbiota and inflammatory disease. Curr Opin Immunol. 2011;23(4):473–80.
Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326(5960):1694–7.
Zraik IM, Heß-Busch Y. Management von nebenwirkungen der chemotherapie und deren langzeitfolgen [management of chemotherapy side effects and their long-term sequelae]. Urologe A. 2021;60(7):862–71. https://doi.org/10.1007/s00120-021-01569-7.
Wallington M, et al. 30-day mortality after systemic anticancer treatment for breast and lung cancer in England: a population-based, observational study. Lancet Oncol. 2016;17:1203–16.
Li H, He J, Jia W. The influence of gut microbiota on drug metabolism and toxicity. Expert Opin Drug Metab Toxicol. 2016;12:31–40.
Feuerstadt P, Louie TJ, Lashner B, Wang EEL, Diao L, Bryant JA, Sims M, Kraft CS, Cohen SH, Berenson CS, et al. SER-109, an oral microbiome therapy for recurrent clostridioides difficile infection. N Engl J Med. 2022;386(3):220–9. https://doi.org/10.1056/NEJMoa2106516.
Bailly C. Irinotecan: 25 years of cancer treatment. Pharmacol Res. 2019;148: 104398. https://doi.org/10.1016/j.phrs.2019.104398.
Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342(6161):967–70.
Hofseth LJ, Hebert JR, Chanda A, et al. Early-onset colorectal cancer: initial clues and current views. Nat Rev Gastroenterol Hepatol. 2020;17(6):352–64. https://doi.org/10.1038/s41575-019-0253-4 (Epub 2020 Feb 21).
Lin XB, Dieleman LA, Ketabi A, Bibova I, Sawyer MB, Xue H, et al. Irinotecan (CPT-11) chemotherapy alters intestinal microbiota in tumour bearing rats. PLoS ONE. 2012;7:e39764. https://doi.org/10.1371/journal.pone.0039764.
Wang L, Wang R, Wei GY, Wang SM, Du GH. Dihydrotanshinone attenuates chemotherapy-induced intestinal mucositis and alters fecal microbiota in mice. Biomed Pharmacother. 2020;128: 110262. https://doi.org/10.1016/j.biopha.2020.110262.
Lian Q, Xu J, Yan S, Huang M, et al. Chemotherapy-induced intestinal inflammatory responses are mediated by exosome secretion of double-strand DNA via AIM2 inflammasome activation. Cell Res. 2017;27(6):784–800. https://doi.org/10.1038/cr.2017.54.
Stringer AM. Interaction between host cells and microbes in chemotherapy-induced mucositis. Nutrients. 2013;5:1488–99. https://doi.org/10.3390/nu5051488.
Ma Y, Peng X, Yang J, Giovanni V, Wang C. Impacts of functional oligosaccharide on intestinal immune modulation in immunosuppressive mice. Saudi J Biol Sci. 2020;27(1):233–41. https://doi.org/10.1016/j.sjbs.2019.08.019 (Epub 2019 Aug 27).
Logan RM, Stringer AM, Bowen JM, et al. The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: pathobiology, animal models and cytotoxic drugs. Cancer Treat Rev. 2007;33(5):448–60. https://doi.org/10.1016/j.ctrv.2007.03.001.
Frosali S, Pagliari D, Gambassi G, et al. How the intricate interaction among toll-like receptors, microbiota, and intestinal immunity can influence gastrointestinal pathology. J Immunol Res. 2015;2015: 489821.
Xie Y, Hu F, Xiang D, Lu H, Li W, Zhao A, Huang L, Wang R. The metabolic effect of gut microbiota on drugs. Drug Metab Rev. 2020;52(1):139–56. https://doi.org/10.1080/03602532.2020.1718691.
Ervin SM, Ramanan SV, Bhatt AP. Relationship between the gut microbiome and systemic chemotherapy. Dig Dis Sci. 2020;65:874–84. https://doi.org/10.1007/s10620-020-06119-3.
Gallotti B, Galvao I, Leles G, Quintanilha MF, Souza RO, Miranda VC, et al. Effects of dietary fibre intake in chemotherapy-induced mucositis in murine model. Br J Nutr. 2020;126:853–64. https://doi.org/10.1017/S0007114520004924.
Cani PD, Jordan BF. Gut microbiota-mediated inflammation in obesity: a link with gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 2018;15:671–82. https://doi.org/10.1038/s41575-018-0025-6.
Morkunas E, Skieceviciene J, Kupcinskas J. The impact of modulating the gastrointestinal microbiota in cancer patients. Best Pract Res Clin Gastroenterol. 2020;48–49: 101700. https://doi.org/10.1016/j.bpg.2020.101700).
Markowiak-Kopeć P, Śliżewska K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients. 2020;12(4):1107. https://doi.org/10.3390/nu12041107.
Pollet RM, D’Agostino EH, Walton WG, Xu Y, Little MS, Biernat KA, et al. An atlas of beta-glucuronidases in the human intestinal microbiome. Structure. 2017;25:967–77. https://doi.org/10.1016/j.str.2017.05.003.
Creekmore BC, Gray JH, Walton WG, Biernat KA, Little MS, Xu Y, et al. Mouse gut microbiome-encoded beta-glucuronidases identified using metagenome analysis guided by protein structure. mSystems. 2019;4:00452–519. https://doi.org/10.1128/mSystems.00452-19.
Wallace BD, Roberts AB, Pollet RM, Ingle JD, Biernat KA, Pellock SJ, et al. Structure and inhibition of microbiome beta-glucuronidases essential to the alleviation of cancer drug toxicity. Chem Biol. 2015;22:1238–49. https://doi.org/10.1016/j.chembiol.2015.08.005.
Forsgård RA, Marrachelli VG, et al. Chemotherapy-induced gastrointestinal toxicity is associated with changes in serum and urine metabolome and fecal microbiota in male Sprague-Dawley rats. Cancer Chemother Pharmacol. 2017;80(2):317–32. https://doi.org/10.1007/s00280-017-3364-z (Epub 2017 Jun 23).
Manhart N, Vierlinger K, Spittler A, Bergut Microbiotaeister H, Sautner T, Roth E. Oral feeding with glutamine prevents lymphocyte and glutathione depletion of Peyer’s patches in endotoxemic mice. Ann Surg. 2001;234(1):92–7. https://doi.org/10.1097/00000658-200107000-00014.
Bowen JM, Stringer AM, Gibson RJ, Yeoh AS, Hannam S, Keefe DM. VSL#3 probiotic treatment reduces chemotherapy-induced diarrhea and weight loss. Cancer Biol Ther. 2007;6:1449–54. https://doi.org/10.4161/cbt.6.9.4622.
Sistigu A, Viaud S, Chaput N, Bracci L, Proietti E, Zitvogel L. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin Immunopathol. 2011;33(4):369–83. https://doi.org/10.1007/s00281-011-0245-0.
Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34(2):336–44. https://doi.org/10.1002/eji.200324181.
Viaud S, Saccheri F, Mignot G, et al. Thcoccus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-indcience. Science. 2013;342(6161):971–6. https://doi.org/10.1126/science.1240537.
Daillère R, Vétizou M, Waldschmitt N, Yamazaki T, et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity. 2016;45(4):931–943. https://doi.org/10.1016/j.immuni.2016.09.009.
Yang Y, Li W, Li Y, Wang Q, Gao L, Zhao JJ. Dietary Lycium barbarum polysaccharide induces Nrf2/ARE pathway and ameliorates insulin resistance induced by high-fat via activation of PI3K/AKT signaling. Oxidative Med Cell Longev. 2014;2014:145641–52. https://doi.org/10.1155/2014/145641.
Puertollano MA, Puertollano E, Cienfuegos GAD, Pablo MAD. Dietary antioxidants: immunity and host defense. Curr Top Med Chem. 2011;11:1752–66. https://doi.org/10.2174/156802611796235107.
Hx A, Swa C, Cca B. Hypoglycemic polysaccharides from Auricularia auricula and Auricularia polytricha inhibit oxidative stress, NF-κB signaling and proinflammatory cytokine production in streptozotocin-induced diabetic mice - ScienceDirect. Food Sci Human Wellness. 2021;10(1):87–93. https://doi.org/10.1016/j.fshw.2020.06.001.
Xu L, Mayila T, Wang J. Explore the effects of Fe3O4 nanoparticles and oxidative stress and neuroinflammatory responses on the gut microbiota based on a Parkinson rat model. J Nanosci Nanotechno. 2021;21:1176–83. https://doi.org/10.1166/JNN.2021.18636.
García-González AP, Ritter AD, Shrestha S, Andersen EC, Yilmaz LS, Walhout AJM. Bacterial metabolism affects the C. elegans response to cancer chemotherapeutics. Cell. 2017;169(3):431–41. https://doi.org/10.1016/j.cell.2017.03.046.
Justino PF, Melo LF, Nogueira AF, Morais CM, Mendes WO, Franco AX, et al. Regulatory role of lactobacillus acidophilus on inflammation and gastric dysmotility in intestinal mucositis induced by 5-fluorouracil in mice. Cancer Chemother Pharmacol. 2015;75:559–67.
Stringer AM, Gibson RJ, Bowen JM, Logan RM, Yeoh AS, Keefe DM. Chemotherapy-induced mucositis: the role of gastrointestinal microflora and mucins in the luminal environment. J Support Oncol. 2007;5:259–67.
Hamouda N, Sano T, Oikawa Y, et al. Apoptosis, dysbiosis and expression of inflammatory cytokines are sequential events in the development of 5-fluorouracil-induced intestinal mucositis in mice. Basic Clin Pharmacol Toxicol. 2017;121(3):159–68. https://doi.org/10.1111/bcpt.12793.
Nomoto K, Yokokura T, Mitsuyama M, Yoshikai Y, Nomoto K. Prevention of indigenous infection of mice with Escherichia coli by nonspecifific immunostimulation. Antimicrob Agents Chemother. 1992;36(2):361–7.
Rhee KJ, Sethupathi P, Driks A, Lanning DK, Knight KL. Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire. J Immunol. 2004;172(2):1118–24.
Gustafsson BE. The physiological importance of the colonic microflflora. Scand J Gastroenterol Suppl. 1982;77:117–31.
Lyte M. Microbial endocrinology: an evolution-based shared mechanism determining microbiota’s influence on health and disease[J]. Else Kroner-Fresenius Symposia. 2013;4:53–8.
Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh AS, Hamilton J, Keefe DM. Gastrointestinal microflora and mucins may play a critical role in the development of 5-Fluorouracil-induced gastrointestinal mucositis. Exp Biol Med (Maywood). 2009;234(4):430–41. https://doi.org/10.3181/0810-RM-301 (Epub 2009 Jan 28).
Chen H, Xu C, Zhang F, et al. The gut microbiota attenuates muscle wasting by regulating energy metabolism in chemotherapy-induced malnutrition rats. Cancer Chemother Pharmacol. 2020;85(6):1049–62. https://doi.org/10.1007/s00280-020-04060-w (Epub 2020 May 15).
Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–79.
Shen S, Lim G, et al. Gut microbiota is critical for the induction of chemotherapy-induced pain. Nat Neurosci. 2017;20(9):1213–6. https://doi.org/10.1038/nn.4606 (Epub 2017 Jul 17).
He Y, Fu L, Li Y, et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8+ T cell immunity. Cell Metab. 2021;33(5):988-1000.e7. https://doi.org/10.1016/j.cmet.2021.03.002.
Gui QF, Lu HF, Zhang CX, Xu ZR, Yang YH. Well-balanced commensal microbiota contributes to anti-cancer response in a lung cancer mouse model. Genet Mol Res. 2015;14(2):5642–51. https://doi.org/10.4238/2015.May.25.16.
Lhchang LY, Ou CC. D-methionine alleviates cisplatin-induced mucositis by restoring the gut microbiota structure and improving intestinal inflammation. Ther Adv Med Oncol. 2019;11:1758835918821021. https://doi.org/10.1177/1758835918821021.
Ren X, Lei L, Liu P, et al. Polysaccharide extracted from Enteromorpha ameliorates Cisplastin-induced small intestine injury in mice[J]. J Func Foods. 2018. https://doi.org/10.1016/j.jff.2018.08.023.
Bingula R, Filaire M, Radosevic-Robin N, et al. Characterisation of gut, lung, and upper airways microbiota in patients with non-small cell lung carcinoma: study protocol for case-control observational trial. Medicine (Baltimore). 2018;97(50): e13676. https://doi.org/10.1097/MD.0000000000013676.
Spferrone CR, Huttenhower C, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357(6356):1156–60. https://doi.org/10.1126/science.aah5043.
Panebianco C, Adamberg K, et al. Influence of gemcitabine chemotherapy on the microbiota of pancreatic cancer xenografted mice. Cancer Chemother Pharmacol. 2018;81(4):773–82. https://doi.org/10.1007/s00280-018-3549-0.
Kim C, Park J, Kim M. Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw. 2014;14:277–88.
Loman BR, Jordan KR, et al. Chemotherapy-induced neuroinflammation is associated with disrupted colonic and bacterial homeostasis in female mice. Sci Rep. 2019;9(1):16490. https://doi.org/10.1038/s41598-019-52893-0.
Su J, Li D, Chen Q, et al. Anti-breast cancer enhancement of a polysaccharide from spore of ganoderma lucidum with paclitaxel: suppression on tumor metabolism with gut microbiota reshaping. Front Microbiol. 2018;17(9):3099. https://doi.org/10.3389/fmicb.2018.03099.
Chattopadhyay I, Nandi D, Nag A. The pint- sized powerhouse: illuminating the mighty role of the gut microbiome in improving the outcome of anti- cancer therapy. Semin Cancer Biol. 2021;70:98–111. https://doi.org/10.1016/j.semcancer.2020.07.012.
Yi Y, Shen L, Shi W, Xia F, Zhang H, et al. Gut microbiome components predict response to neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer: a prospective. Longitudinal Study Clin Cancer Res. 2021;27(5):1329–40. https://doi.org/10.1158/1078-0432.CCR-20-3445.
Zhou H, Suo J, Zhu J. Therapeutic relevance of human microbiota and lung cancer. Zhongguo Fei Ai Za Zhi. 2019;22(7):464–9. https://doi.org/10.3779/j.issn.1009-3419.2019.07.09.
Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–9.
Jacouton E, Chain F, Sokol H, Langella P, Bermudez- Humaran LG. Probiotic strain lactobacillus casei BL23 prevents colitis-associated colorectal cancer. Front Immunol. 2017;8:1553.
Yazdi MH, Mahdavi M, Setayesh N, Esfandyar M, Shahverdi AR. Selenium nanoparticle-enriched Lactobacillus brevis causes more efficient immune responses in vivo and reduces the liver metastasis in metastatic form of mouse breast cancer. Daru. 2013;21(1):33.
Aragón F, Carino S, Perdigón G, De MorenoDe LeBlanc A. The administration of milk fermented by the probiotic Lactobacillus casei CRL 431 exerts an immunomodulatory effect against a breast tumour in a mouse model. Immunobiology. 2014;219:457–64.
Lakritz JR, Poutahidis T, Levkovich T, Varian BJ, Ibrahim YM, Chatzigiagkos A, et al. Beneficial bacteria stimulate host immune cells to counteract dietary and genetic predisposition to mammary cancer in mice. Int J Cancer. 2014;135:529–40.
Stringer AM, Gibson RJ, Yeoh AS, Hannam S, Keefe DM. VSL#3 probiotic treatment reduces chemotherapy-induced diarrhea and weight loss. Cancer Biol Ther. 2007;6(9):1449–54.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, L., Bai, Y., Xiang, L. et al. Interaction between gut microbiota and tumour chemotherapy. Clin Transl Oncol 24, 2330–2341 (2022). https://doi.org/10.1007/s12094-022-02919-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02919-3